This week’s blog is part 1 of 2, where Water Hygiene Centre looks at TVC’s and explains what they are, including organisms and method of analysis.
Introduction to Microbiology
Aerobic colony counts, heterotrophic plate counts and total viable cell [TVC] counts are different names for essentially the same thing: A quantitative assessment of the number of living heterotrophic microorganisms in a sample of water.
They are a common method of monitoring the number of heterotrophic bacteria in water samples. In simple terms, the test is performed by combining a sample of water with a source of nutrients and placing them in an environment conducive to microbial growth. After a period of time the number of microbial colonies that have formed is counted giving a numerical reading.
For the purpose of this blog we refer to this method of analysis as TVC; as this is currently the most commonly used term in the field of building water systems management.
Heterotrophic Microorganisms
The “heterotrophs” are a large group of organisms that all use organic carbon as their main energy source. Unlike the “autotrophs” that fix their own carbon from the gases in the atmosphere [e.g. photosynthetic plants], heterotrophs source their carbon from other forms of life, either from living plants, animals etc. or from the material left behind when those organisms die.
Humans are heterotrophs sourcing the carbon needed to grow, repair and produce energy from the food that we eat. As well as all animals, the group also includes macroscopic fungi and a wide range of microorganisms including moulds, yeasts and some bacteria. Heterotrophic microorganisms are present in most drinking water supplies, including bottled water, to varying degrees.
The colony count
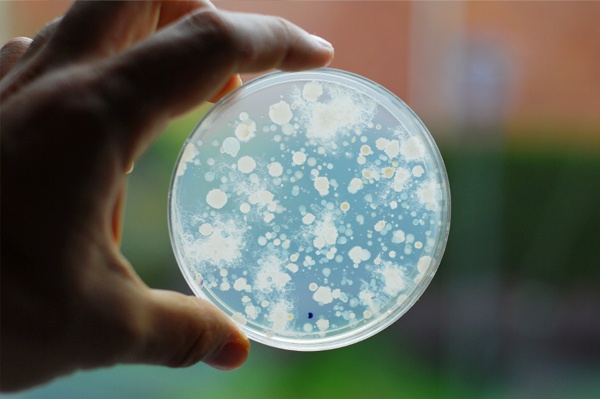
In the UK the most common methods for the enumeration of heterotrophic bacteria remain the pour-plate and spread-plate methods. Both methods are performed in petri dishes by adding test volumes of water to a general purpose agar-based growth media, such as yeast extract agar [YEA], a source of organic carbon.
The dishes are then placed in a controlled temperature environment [i.e. an incubator] for a prescribed amount of time before counting the colonies that have developed. Each colony that develops represents a living organism, known as a “viable cell” and hence the name total viable cell [TVC] counts.
TVC counts are recorded as colony forming units [CFU] where each colony counted on the plate equates to one CFU. Counts are reported as CFU per millilitre of sample tested [CFU/ml]. Counts above 300 CFU on a single petri-dish are considered unreliable due to competition for space and nutrients.
In a similar way, any plate which contains an organism or organisms that have spread across the surface of the agar will also be considered unreliable. For this reason, samples of water that are expected to return results of greater than 300 CFU/ml require dilutions to be carried out on them. Dilutions are done at the laboratory under aseptic technique and should not be attempted at source!
Incubation
Once plated, the incubation of the samples is critical to the outcome of the test. Incubation at different temperatures for different lengths of time will provide varying results. It is therefore vitally important that incubation parameters are standardised in order for results between tests to be comparable. The current standards can be summarised, as follows:
Incubation at 22°C [68 ±4hrs] aka “cold plate count”
Used by water supply companies for compliance monitoring of drinking water supplies and also widely advocated in guidance as a means of monitoring trends in water quality in the built environment. Counts made after incubation at 22°C are indicative of the number of all type of bacteria present at normal environmental temperature, and therefore mainly from soil and vegetation.
Incubation at 37°C [44 ±4hrs] aka “hot plate count”
Recently dropped from Schedule 2 of the Water Supply [Water Quality] Regulations, colony counts performed at 37°C are no longer required to be carried out by water companies as part of routine compliance monitoring. However, the analysis remains valuable in particular circumstances and is widely referred to in guidance, some published as recently as 2015 and 2016. Hot plate counts encourage the growth of bacteria that thrive at body temperature and which may be enteric bacteria [i.e. of faecal origin].
Incubation at 30°C [44 ±4hrs]
Incubation at 30°C is used for tests carried out during routine monitoring of cooling towers and, in healthcare, on final rinse waters from washer disinfectors for assurance purposes. This temperature is used as it is considered most likely to be conducive to the growth of organisms favouring the conditions found within the systems being tested.
Sampling Plan
Before any sampling takes place there should always be a sampling plan to ensure that the information gathered is meaningful and relevant to a specific purpose. The sampling plan should define the aim and objectives of the sampling programme and describe how these will be achieved.
The plan will determine what type of samples are required, where they are to be taken, how many samples are required and how many and how often. More information on how to plan a sampling programme can be found in BS 8554:2015 and the Standing Committee of Analysts’ “The Microbiology of Drinking Water (2010) - Part 2 – Practices and procedures for sampling”.
Samples should only be collected by competent individuals who have been fully briefed on the sampling plan. Samples are to be collected aseptically in sterile containers containing an appropriate quantity of neutralising agent for any disinfectant in use. Once samples have been collected they should be stored in refrigerated conditions at 5[±3] °C and delivered to the laboratory as soon as possible and within 24-hours after collection.
Sampling plans may form part of a Water Safety Plan [WSP] designed to promote good practices in building water systems management.
Part 2 will look at when to use TVC’s and what they can tell us about our building water systems.
For more information on how Water Hygiene Centre can assist with sampling requirements, water safety plans and other aspects of water safety, visit our services page.
Editors Note: The information provided in this blog is correct at date of original publication - January 2018.
© Water Hygiene Centre 2019